By Prachi Patel / April 2013
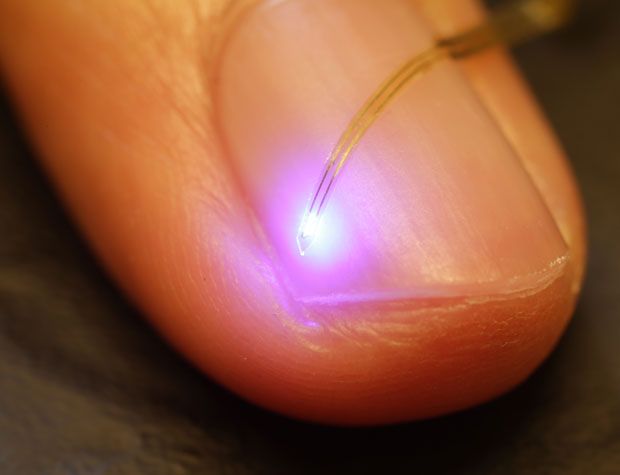
But now, a new ultrathin, flexible device laden with light-emitting diodes and sensors, both the size of individual brain cells, promises to make optogenetics completely wireless. The 20-micrometer-thick device can be safely injected deep into the brain and controlled and powered using radio-frequency signals. Its developers say the technology could also be used in other parts of the body, with broad implications for medical diagnosis and therapy.
In optogenetics, scientists genetically modify neurons to make them sensitive to particular wavelengths of light. Shining light on the altered neurons turns them on or off, allowing scientists to control specific brain circuits and change animal behavior.
Michael Bruchas, a neurobiologist at Washington University, in St. Louis, wanted to develop a wireless alternative to the fiber-optic approach. “Wires limit the animal’s natural behavior,” he says. “We couldn’t explore some experiments with the animals tethered, say, if we want two animals interacting or want them to be in a naturalistic environment.” So he teamed up with John Rogers at the University of Illinois at Urbana-Champaign to design a new system based on extremely thin semiconductor devices. The researchers published their results in this week’s issue of the journal Science.
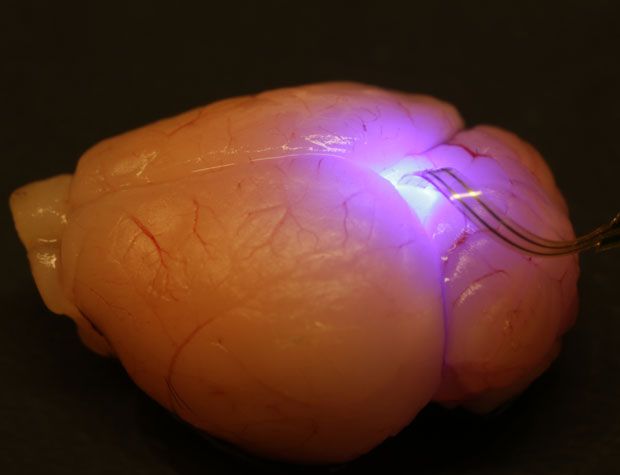
The technique for making the membranous devices is not new. Developed a few years ago in Rogers’s lab, it involves growing stacks of thin semiconductor films, peeling them off one at a time with a rubber stamp, and transferring them to plastic substrates.
Scientists could use the multifunctional system to stimulate and sense the brain in a variety of ways, Bruchas explains. The microelectrode can measure the electrical signals produced by neurons, and it can also be used to stimulate them. The photodiodes ensure that the LEDs are working, but they can also be used to detect light signals generated by neurons that have been genetically modified to make certain fluorescent proteins.
The micro-LEDs, which have dimensions comparable to individual neurons, could trigger individual neurons, unlike the fiber-optic implants typically used in optogenetics, which are four times as wide. The researchers could also combine different-colored LEDs on the same device and use them to simultaneously control neurons that have been engineered to react to different colors. Such multiplexing would allow neuroscientists to analyze brain circuits more precisely, Bruchas says. Finally, the temperature sensor monitors the heat generated by the LEDs to prevent the tissue from overheating.
When the researchers placed the device—which connects to an RF power module mounted on the animal’s head—inside the brains of living mice, it caused no inflammation or infection. To test the system’s ability to alter animal behavior, the researchers embedded it near a particular group of neurons that they had genetically altered to release dopamine when cued with light. The neurochemical dopamine is involved in the body’s “rewards” system, such as with food or sex, and it plays a part in several addictive drugs.
In this experiment, the mice were placed in a maze. When the animals reached a particular place in the maze, the researchers wirelessly pulsed the LEDs on and off, triggering the release of dopamine. The animals quickly learned to go to that spot for the pleasure sensation.
“The device illustrates the integration of miniaturized semiconductor devices deep within tissue, be it brain or the heart or any other organ,” Rogers says. More-sophisticated silicon-based microcircuits could be implanted in the future, he says, paving the way for applications in medical diagnosis, monitoring, and treatment.
The work “shows how miniaturization and systems integration guide us toward novel devices and applications,” says Thomas Stieglitz, a professor in the department of microsystems engineering at the University of Freiburg, in Germany. Stieglitz and his colleagues are also trying to develop a wireless, possibly biodegradable, implant for optogenetics.
“The ability to perform wireless stimulation and sensing is going to open up new doors for behavioral neuroscience, allowing the study of more complex behaviors, such as social interaction and mating behaviors,” says Kay Tye, a professor of neuroscience at MIT.
About the Author
Prachi Patel is a contributing editor to IEEE Spectrum. In February 2013, she reported on another optogenetics advance.http://spectrum.ieee.org/biomedical/devices/injectable-optoelectronics-for-brain-control/